How Are PRP Tubes Manufactured?
Many clients request short delivery times when ordering PRP tubes, which often indicates a lack of awareness regarding the complexity of their manufacturing process. Unlike standard blood collection tubes used solely for testing, PRP tubes are high-precision medical devices intended for injectable procedures. Their production demands a far more rigorous and intricate approach.
PRP tubes typically carry a two-year shelf life, but actual longevity is highly dependent on the material. Tubes made from PET material, for example, experience a significantly shorter vacuum retention period—usually around one year. This is due to PET’s inherent property of retaining moisture but not vacuum. In contrast, glass tubes excel in both water and vacuum retention. When properly cleaned and dried, glass tubes can maintain vacuum stability for up to two years. However, their main drawback is fragility. A broken tube not only compromises the sample but also poses a safety risk. Therefore, the choice between glass and plastic should be based on specific application needs. We generally recommend using PRP tubes of any material within one year of production.
Step-by-Step Production Process:
Gel Filling → Pre-Centrifugation → Labeling → Additive Dispensing → Stopper Placement → Vacuum Sealing → Quality Inspection → Packaging → Boxing → Shrink Wrapping → Cartoning → Sterilization → Completion
Detailed Manufacturing Stages:
1, Separation Gel Filling

A specialized separation gel is dispensed into each PRP tube. This gel is critical for effectively separating plasma from red blood cells during centrifugation. Automated filling systems ensure precise and uniform gel distribution in every tube.
2, Pre-Centrifugation Testing
Before sealing, tubes undergo pre-centrifugation to verify that the gel settles evenly at the bottom. This step confirms product consistency and performance reliability.
3, Labeling
An automated labeling system applies labels with accurate, legible information, ensuring correct positioning and adherence.
4, Additive Dispensing

A precision liquid-dispensing system injects anticoagulants or other reagents into each tube according to predefined specifications.
5, Vacuum Sealing
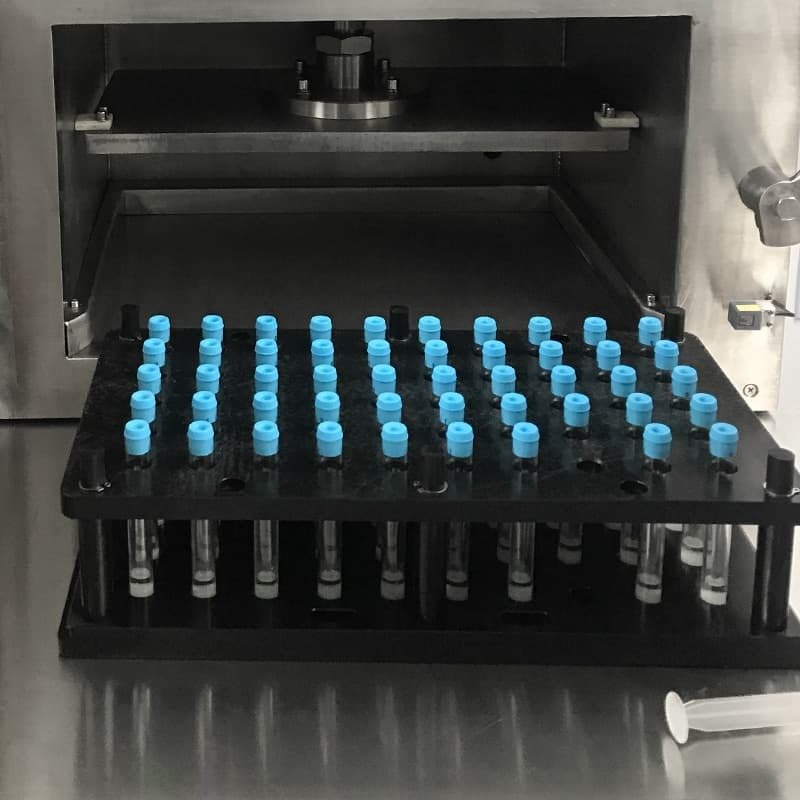
After additive dispensing, tubes enter a sealed chamber where a preset vacuum level is applied. The tubes are simultaneously sealed under vacuum to maintain sterility and stability.
6, Quality Sampling
Random samples are taken for rigorous quality checks, including sterility testing, vacuum verification, gel distribution uniformity, and label adhesion.
7, Packaging
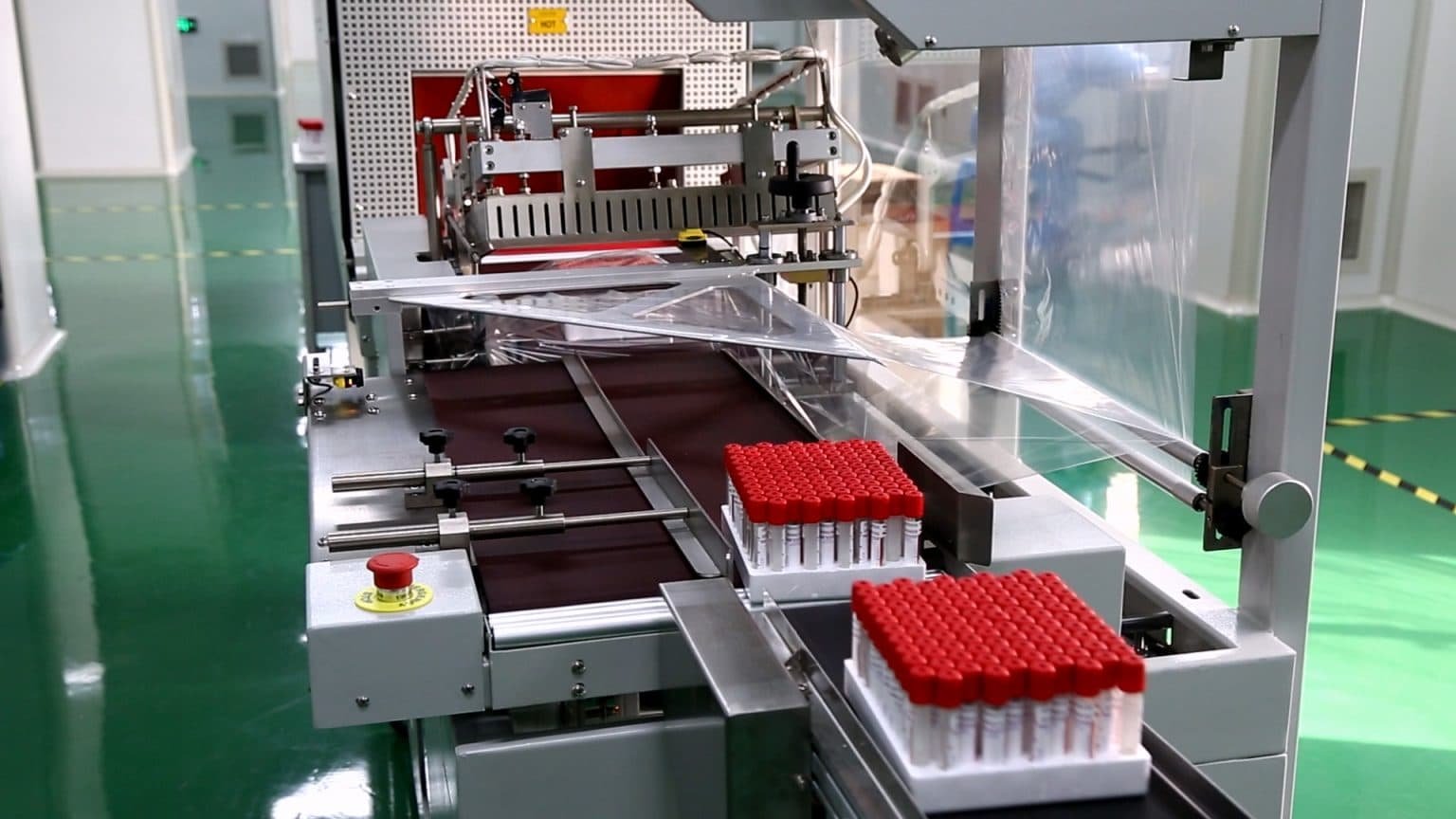
Qualified tubes are aseptically packaged to preserve sterility throughout storage and transit.
8, Sterilization
Packaged products are sent to a certified sterilization provider. Gamma irradiation (Co60) is commonly used to ensure complete elimination of microorganisms.
9, Shipping
Once sterilized, the PRP tubes are ready as finished products and can be dispatched.
10, Key Consideration:
While additives and tube dimensions form the basic structure of PRP tubes, it is the sterilization process that defines their safety and efficacy. Only thoroughly sterilized tubes qualify as true PRP tubes. Without proper sterilization, they remain ordinary blood collection tubes—with a significant gap in both value and safety.